Bioquell SeQure
The Bioquell SeQure is the fixed and compact wall-mounted biodecontamination system that eradicates the bioburden of microorganisms and other contaminants threatening your research and production facility.
Ideal for:
- Pass-Throughs & Material Airlocks
- Lab & Room Decontamination
- Spaces Smaller Than 150 Cubic Meters
- Viral & Bacterial Vector Work
- Biosecurity
- Small Research Areas
Watch Video 
WHY CHOOSE THE Bioquell SeQure

Productive
Fast, effective automated cycles for high throughput
Adaptable
Ideal system for retrofitting or new construction; mounting ability on nearly any wall surface

Assured
Full GMP compliance; automatic cycle-prediction technology for swift validation
Efficient
Push-button decontamination of every exposed surface; reduction of manual labor and the risk of error with spray-and-wipe methods

Integrated
Wall mounting with connectivity to your building management system or SCADA via Modbus protocol
Rapid
Automated, validated cycles and distinctive design for installation and validation in less than one week


Watch Bioquell SeQure Video
Preview Key Benefits and Features
APPLICABLE SOLUTIONS
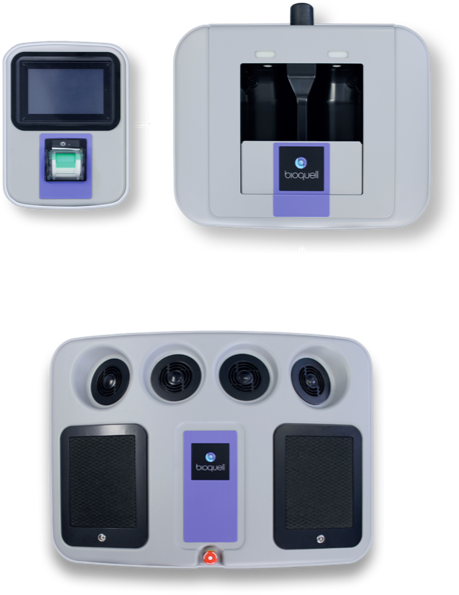
COMPONENTS
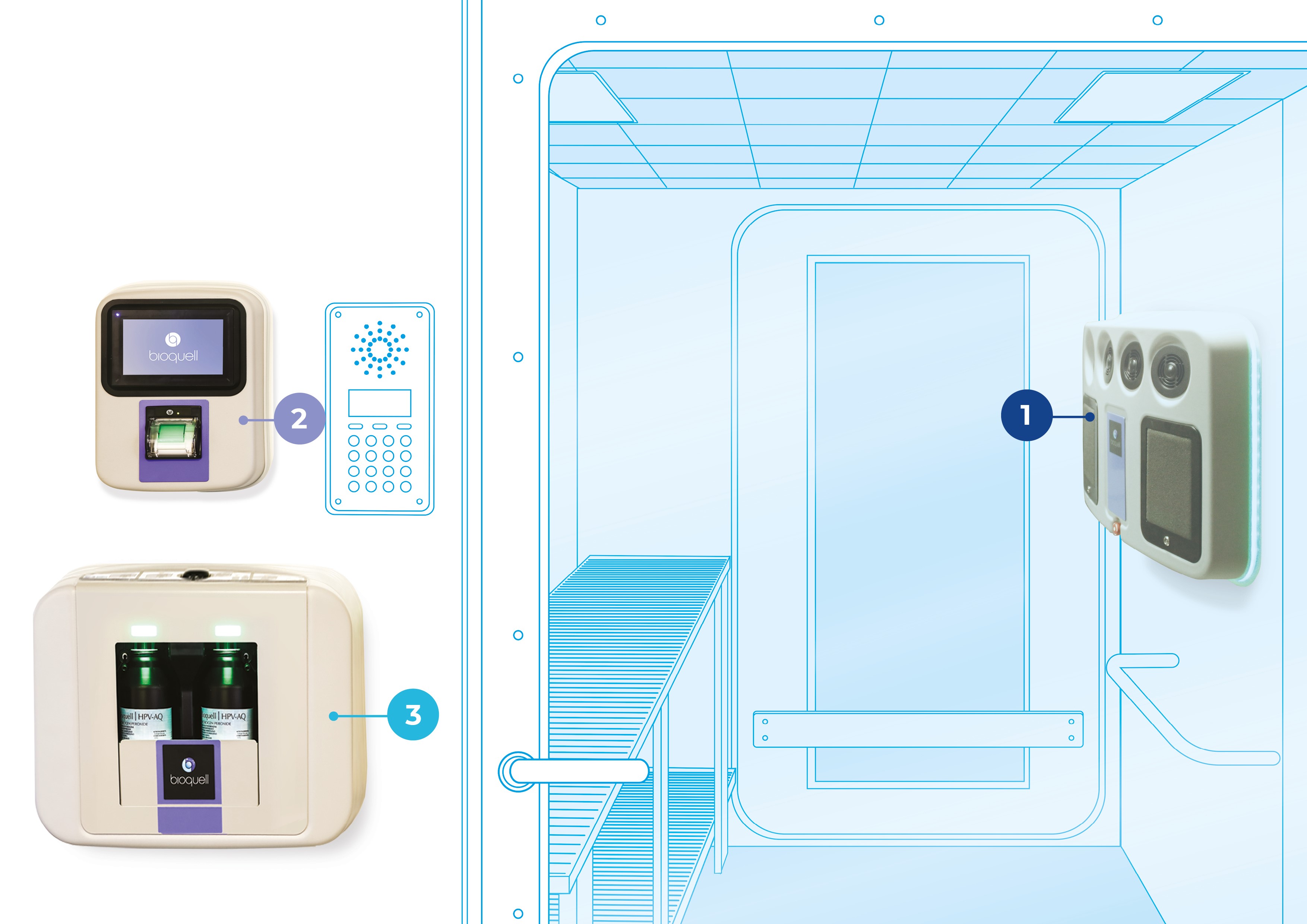
BIOQUELL SEQURE COMPONENTS

- 4 integrated fans distribute vapor dynamically
- Automated cycle prediction technology
- Operates at room temperature conditions, safe for heat-sensitive materials

- Easy-to-use, color touchscreen
- Password Protected programmed decontamination cycles

- Minimizes peroxide wastage with no decanting
- Houses 2 H2O2 bottles (950ml each)
CONSUMABLES & ACCESSORIES

Bioquell Hydrogen Peroxide
Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell SeQure system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology

Bioquell’s proprietary high-purity aqueous 35% solution for use with the Bioquell SeQure system achieves 99.9999% deactivation of pathogens and a 6-log kill with full traceability and auditing through RFID label technology
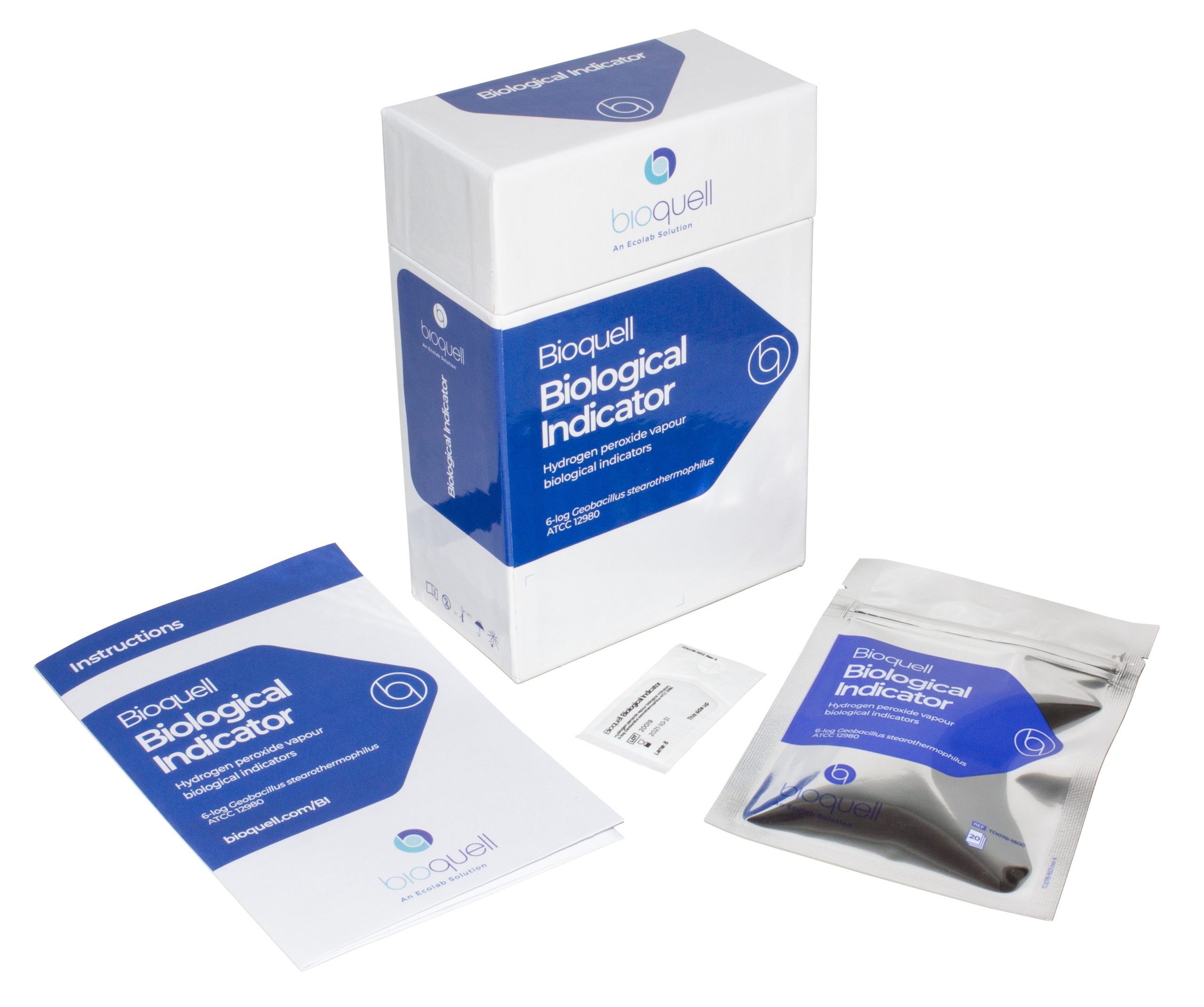
Our individually crafted biological indicators made from Geobacillus stearothermophilus endospores ensure reliable results when seeking to validate a 6-log reduction of bioburden from the decontamination process

For when your HVAC system cannot help remove Hydrogen Peroxide Vapor, our slim wall- or ceiling-mounted unit quickens your decontamination cycles and communicates with the Bioquell SeQure system for faster turnaround

The Bioquell Room CI is our chemical indicator developed for rooms 25m3 and larger. Place one or multiple indicators in the room you are performing Bioquell bio-decontamination to see your expected results in real-time. The color changing strip printed on the CI has a reactive dye specifically tailored for monitoring Bioquell’s bio-decontamination process, allowing you to see the results as they occur.
VALIDATION
Bioquell SeQure system components are wall-mounted inside or outside of your workspace for a minimal footprint that maximizes your working area, allowing for greater productivity.
Whether installed in a new facility or retrofitted into an existing workspace, each component integrates quickly to minimize downtime while you upgrade to the latest decontamination available. Full installation and validation typically take less than one week.
Once your installation and validation are complete, your lab will secure the potential benefits from the reduced financial impact of contamination events that can occur and the opportunity costs lost to manual biodecontamination methods.
FAQs
Will this offer 21-CFR Part 11 compliance?
Yes, we are able to offer an optional data logger for this purpose.
Will installation and validation of the Bioquell SeQure disrupt my workflow?
No. Depending on the volume of the area and the level of equipment contained therein, installation and retrofitting of the Bioquell SeQure can be completed and validated within a week. Our technicians can work during non-operating hours if possible and permitted by the client. Specific timeframes will be given for each project.
Can the Bioquell SeQure connect to my Building Management System?
Yes. Our expert technicians will perform a walkthrough of your cleanroom to ensure your systems are compatible with the Bioquell SeQure for integrated HVAC and automated operation. What’s more, Bioquell experts provide continued support for systems that are already in place, ensuring full BMS connectivity and solutions for the lifetime of your system.
How compact is the Bioquell SeQure?
Vaporizer: 800 x 580 x 160 mm (31.5 x 22.8 x 6.3 in)
Bottle Module: 520 x 550 x 140 mm (20.5 x 21.7 x 5.5 in)
Wired Control Unit: 260 x 350 x 95 mm (10.2 x 13.8 x 3.7 in)
Contact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Bioquell Inc.
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
solutions@bioquell.com










