Hospital Pharmacy and Compounding
Safety and quality concerns of medications have healthcare providers opting to bring drug compounding under their own control. Bioquell’s solutions ensure your hospital pharmacy and compounding operations can retain their integrity while complying with the highest standards, ensuring your patients and staff are safe.
Applications
Compounding
Compliance
Cost Savings
Patient and Staff Safety

Compounding
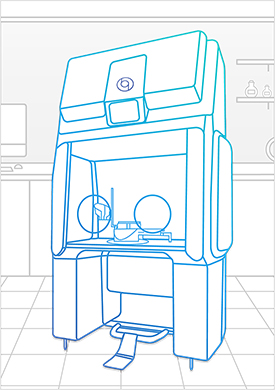
Bioquell offers a modular and unique containment system with built-in hydrogen peroxide vapor decontamination that can be used for non-sterile and sterile compounding, and can be vented for Hazardous Drugs (HDs) preparation utilizing negative pressure. Nearly any compounding operation can be performed comfortably and safely with the Bioquell Qube.
SYSTEMS AND SERVICES
Bioquell’s options for the hospital pharmacy and drug compounding decontamination provide advanced Hydrogen Peroxide Vapor technology in your clean spaces and containment isolators. You can feel confident that your patients, staff and compounded drugs, whether sterile or non-sterile, are protected from contaminants.
Bioquell Qube for Healthcare
You can create a secure aseptic processing workspace to prepare individual patient prescriptions while meeting the latest regulations, and protecting your patients and staff with the Bioquell Qube. This configurable isolator comes integrated with Bioquell’s Hydrogen Peroxide Vapour technology.
Learn MoreBioquell SeQure
The Bioquell SeQure provides a fixed solution with advanced controls to ensure critical areas can be quickly and easily decontaminated to the fullest extent, emphasizing patient and staff safety.
Learn MoreRapid Bio-Decontamination Service (RBDS)
Infection control priorities can shift at any moment, requiring an advanced decontamination that UV and aerosolized technologies cannot provide. Bioquell’s Rapid Bio Decontamination Service (RBDS) can be quickly called upon to eliminate pathogens, stop outbreaks, handle emergency response situations and much more for any size area.
Learn MoreBioquell Proactive
The Bioquell Proactive provides a full-time Bioquell technician at your facility to perform daily decontamination with Hydrogen Peroxide Vapor in accordance with your infection control strategy. This service includes staffing, equipment needs and maintenance, consumables, reporting, technology upgrades and more.
Learn MoreContact Us
To learn more about how Bioquell can fit your solution, please contact us.
The Americas
Bioquell Inc.
702 Electronic Dr. Suite 200
Horsham, PA 19044
+1 215 682 0225
solutions@bioquell.com